Jay Horrow, MD, MS, FAHA
- Professor of Anesthesiology, Physiology, and Pharmacology
- Drexel University College of Medicine
- Professor of Epidemiology and Biostatistics
- Drexel University School of Public Health
- Philadelphia, Pennsylvania
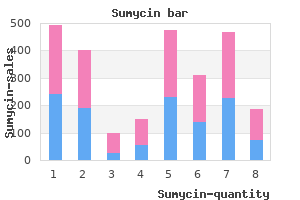
Reduction of Vasogenic Edema, Radioprotection, and Radiosensitization A major cause of morbidity and mortality from brain tumors is the peritumoral, vasogenic edema that can cause mass effect and midline shift and is responsible for much of the morbidity and mortality of brain tumors antibiotic resistance lecture buy discount sumycin 500 mg on line. Most patients treated with bevacizumab had at least a 50% reduction in corticosteroid dose antibiotic resistance why is it a problem order sumycin 250 mg without prescription. The steroid-sparing effect of cediranib persists until the drug is discontinued because of tumor progression treatment for dogs false pregnancy purchase sumycin with paypal. Heretofore, the mainstay of therapy has been corticosteroids, with surgical decompression used if the patient became steroid dependent or steroid toxic, or if there were symptoms and mass effect despite corticosteroids natural treatment for dogs fleas buy sumycin 500mg mastercard. Treatment with hyperbaric oxygen, vitamin E, and other antioxidants yield only modest benefits antimicrobial honey order sumycin with visa. Six complementary modalities (A-F) illustrate the value of separate techniques to measure specific functional aspects of the brain vasculature as it responds to an angiogenic inhibitor. A, T1-weighted gadolinium shows the rapid response and disappearance of contrast enhancement in the left frontal lobe after 1 day and disappearance by day 27. B, A map of the relative microvessel size also shrinks soon after starting therapy. C, Maps of permeability (Ktrans) show an immediate improvement after the first dose of the inhibitor. F, Diffusion tractography shows that the directional water mobility corresponding to fiber pathways (green) in the white matter surrounding the tumor are visualized as the edema clears. A fluorodeoxyglucose positron-emission tomography scan and a single-photon emission computed tomography scan did not reveal any uptake of the radionuclide in the corresponding area of contrast enhancement. Bevacizumab combined with hypofractionated stereotactic irradiation proved safe and well tolerated with evidence of activity. Bevacizumab is generally given at a dosage of 10 mg/kg every 14 days on a 28-day cycle; two infusions constitute one cycle. The maximal tolerable dose is about 15 to 20 mg/kg; the optimal biologic dose is unknown. Highly vascular tumors, such as glioblastoma or metastatic melanoma, sporadically present with spontaneous intracerebral hemorrhage. Indeed, patients with glioblastoma who develop venous thromboembolism can safely undergo anticoagulation while receiving treatment with bevacizumab. The combination of bevacizumab and irinotecan is effective in producing a dramatic response in most patients with malignant glioma as measured by >50% reduction in tumor size (Partial Response; Macdonald Criteria), and 6-month progression-free survival of >50%, much greater than historical controls. It is unclear whether discontinuation causes a "rebound" in angiogenesis and tumor growth. Currently, none of these agents has had similar immediate, dramatic, and sustained responses as bevacizumab, which appears to be the prototype for angiotherapy as penicillin was for antibiotics. The International Brain Tumor Association issued a White Paper on bevacizumab, declaring it the de facto treatment for recurrent glioblastoma worldwide. The cost of bevacizumab, and other molecular targeted agents, is an issue, especially in the current economic crisis and national health care debate. For the individual patient, the cost can be a significant barrier to acquire the drug. Recently, Medicare agreed to consider payments to cover the costs of bevacizumab for patients with glioblastoma. A group of patients respond initially but then progress with a diffuse, nonangiogenic, invasive phenotype. Bevacizumab lacks many of the classic toxicities of cytotoxic chemotherapy such as myelosuppression, alopecia, and immunosuppression. Bevacizumab and other antiangiogenics need to be given under close medical supervision. Bevacizumab should not be given to patients with "bowel disorders" such as diverticulitis. Intracranial hemorrhage is extremely rare, and patients with brain tumors who are receiving bevacizumab can also be safely treated with systemic anticoagulation. Intracerebral hemorrhage is not greater than occurs during the natural history of patients with brain tumors. Bevacizumab and other antiangiogenics act, in part, by normalizing the vascular anatomy and physiology. There is a "normalization window" that explains the time and dose dependency of antiangiogenic drug delivery. The vascular normalization explains the paradoxical synergies of angiotherapy with chemotherapy and radiation therapy because improved oxygenation makes tumor cells chemosensitive and radiosensitive. Large angiosuppressive molecules, such as bevacizumab (149 kD), can be effective because they do not rely on disruption of the blood-brain barrier, but rather are effective within the lumen of the capillary endothelium. Biomarkers would be valuable to monitor and guide therapy, predict relapse, and act as an intermediate end point in clinical trials. For relapse while taking bevacizumab, changing the cytotoxic chemotherapy backbone is an option. In addition to irinotecan, carboplatin, temozolomide, and etoposide have been tried-none with any significant success. Quality of life appears improved in patients treated with bevacizumab, but quantitative and prospective studies are needed. For other malignancies, there are objective data that angiotherapy improves quality of life compared with standard treatment with cytokines (interferon-). Bevacizumab-based regimens appear effective for (recurrent) glioblastomas in all regions of the brain, including deeply seated, unresectable tumors such as those in the thalamus and brainstem and "butterfly" tumors of the corpus callosum. The efficacy of bevacizumab, unlike cytotoxic chemotherapy, does not appear related to patient age or number of prior treatment regimens. Prior treatment with cytotoxic agents may not be a relevant exclusion criterion in the design of trials evaluating antiangiogenic agents. New blood vessels serve to harbor in a protected microenvironment, or vascular niche, proliferating glioma progenitor cells. Angiotherapy synergizes with chemotherapy and radiation therapy by removing the vascular niche and improving oxygenation of tumor cells. The doses generally used, based on a formal, pivotal study,5 are bevacizumab, 10 mg/kg, plus irinotecan every 2 weeks. Anti-invasion therapy that will be as effective as antiangiogenic therapy needs to be developed. Conclusion: Bevacizumab can act like a molecular scalpel to remove the vascular niche, close the angiogenic switch, open the normalization window, and boost immunity, which translates to chemical cytoreduction, decreased vasogenic edema, neurological improvement, and prolonged survival. Wound healing might be less problematic using antiangiogenics with a shorter half-life than bevacizumab, such as sorafenib or sunitinib. Because perforation of the colon can be life-threatening, caregivers need to have a high index of suspicion if a patient develops abdominal pain or other gastrointestinal symptoms. The reason for its rare occurrence and specific location in the posterior cerebral circulation is unknown. For metastatic renal carcinoma, there are objective data that angiotherapy improves quality of life compared with standard treatment with cytokines (interferon-). One of the most pressing and practical dilemmas for patients is the high cost of bevacizumab and other targeted agents, potentially in excess of $100,000 per year. The first is one of a highly angiogenic, focal tumor with mass effect and vasogenic edema. The second is that of an invasive, multifocal tumor with minimal vasogenic edema, resembling "gliomatosis". Each pattern occurs in about one third of treated patients, with the remainder displaying a mixture of the angiogenic and invasive patterns. A 48-year-old woman with a glioblastoma, completely resected 8 months earlier and followed by standard treatment with 60 Gy of involved field radiation and six cycles of temozolomide, developed a local recurrence and was placed on an experimental, proapoptotic targeted therapy, and after 2 months, was started on bevacizumab plus irinotecan. Baseline coronal (A) and axial (B) T1-weighted gadoliniumenhanced magnetic resonance imaging. After three cycles in 2 months, there is a flare of a hypervascular, highly angiogenic tumor (C and D) requiring a second-stage surgery. Note the linear streaks of the neovascularization, necrosis, mass effect, and vasogenic edema associated with the progression of the tumor growth. A, A 40-year-old man presents with a biopsy-verified glioblastoma located in the left temporal lobe. C, Six months after surgery, there is contrast enhancement on the sagittal T1-weighted image with gadolinium, showing laminar infiltration along the ventricular ependymal lining and a posterior temporal, 2. After completing several cycles of temozolomide, he starts antiangiogenic therapy (bevacizumab with irinotecan), and 2 months later (after four cycles of biweekly administration), there is a complete response (D). One month later, the patient has developed clinical side effects linked to the treatment (diarrhea, hypertension, and fatigue) and is given a "drug holiday. In addition, an angiogenic nodule visualized on the T1-weighted image with gadolinium now appears in a new area posterior to the thalamus (N). These "secondary" structures may be of primary importance and include (1) perineuronal satellitosis, (2) perivascular satellitosis, (3) subpial spread, and (4) invasion along the white matter tracts. Tumors respond to molecular therapies either with spontaneous genetic mutations to form or select a drug-resistant, independent clone or upregulation of angiogenic or invasive molecular programs in response to epigenetic and microenvironmental cues, such as hypoxia, proximity to the ventricular system, blood vessels, and cellular infiltrates. As Schilsky noted, "Biologically, the cancer cell is notoriously wily; each time we throw an obstacle in its path, it finds an alternate route that must then be blocked. The model accounts for the variables of dose, duration, and the normalization of the microvascular physiology. Insufficient dose (bottom, tan) or a minimal exposure (left, tan) will result in no effect on the tumor vessels. By contrast, an excessive amount of inhibitor will lead not only to toxicity to normal tissues (top, orange) but also, with time, to risk for excessive "pruning" of vessels, resulting in ischemia. Thus, an optimal biologic dose for an optimal period is necessary to yield a normalization window (center, yellow). The normalization hypothesis is valuable to guide drug discovery and clinical trial development of antiangiogenics against tumors of the central nervous system. Prolonged angiosuppression or an excessive dose can cause ischemia or hypoxia through "pruning" of vessels and lead to the emergence of resistant tumor cells or a more invasive phenotype. The normalization window is time specific, and combining radiation therapy or chemotherapy should occur when the vasculature is normalized. The therapeutic challenge is to maintain a prolonged normalization window, in effect maintaining a dormancy state, indefinitely, limiting the tumor to a barely detectable size,77 that is, "cancer without disease. These interesting findings require validation in larger clinical trials, but circulating endothelial progenitor cells could prove to be a valuable, noninvasive biomarker. A widely available blood test and pharmacodynamic marker would be a breakthrough to enable optimal combination of angiosuppressive therapy, to attack a "moving target," and to enable dose and regimen modification for the individual patient based on quantifiable cellular end points. Moving forward, there are data that angiotherapy not only works better in combination with chemotherapy but also is synergistic with radiotherapy,326-332 immunotherapy,333,334 and, potentially, viral oncolytic therapy. For example, when a bolus of gadolinium travels to the blood vessel in a tumor, some leaks into the interstitial space, whereas the rest remains in the blood vessel as part of the bulk flow; thus, changes in permeability can alter perfusion, and vice versa. That a large (149-kD) molecule such as bevacizumab, which would not be expected to traverse the blood-brain barrier, can effectively suppress tumor growth is noteworthy. Drug resistance (by genetic mutations, biochemical redundancy, vascular co-option, angiogenesis-independent clones, and glioma invasion) remains a formidable therapeutic challenge. In the future, newer drugs,371-386 enhanced drug discovery,387-391 and integrative mathematical modeling392-394 show promise of additional breakthroughs for a second generation of antiangiogenics that can work synergistically with current therapies and turn off the angiogenic switch. That the molecular biology translates into chemical cytoreduction, decreased vasogenic edema, neurological improvement, and prolonged survival is the realization of a long-standing vision. That malignant gliomas are multipleheaded Hydras that can evade even an ehrlichian "silver bullet" signifies that oncologists and neurosurgeons will need to work together to fulfill the folkmanesque concept of "cancer without disease" to permanently tame the beast of glioblastoma. As Dade Lunsford noted, During the last decade of the 20th century and the first years of the 21st century, neurosurgery has been part of an enormous paradigm shift. While we previously concentrated on dealing with the removal or management of structural masses (blood clots. Inhibition of angiogenesis and tumor growth in the brain: suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns or recurrence. According to the Central Brain Tumor Registry of the United States, it is estimated that approximately 20,000 new cases develop each year. The invasiveness of these tumors eliminates the possibility of curative surgical resection. There is vast interest in and consequently multiple research efforts focused on the design of new approaches that will improve drug delivery to brain tumor cells with limited systemic toxicity. This chapter summarizes the methods that have been developed for overcoming the ability of brain tumor cells to remain safe from therapy behind multiple physical and physiologic barriers. Certain molecules, particularly large water-soluble molecules, are prohibited from diffusing through the gaps between these cells. Small, lipophilic molecules can, however, diffuse across the membranes, but they too are limited in distribution by diffusion. Active or facilitated transport is used for substances with low partition coefficients. This type of transport is dependent on ion channels, specific transporters, energy-dependent pumps, and receptor-mediated endocytosis. Glucose, amino acids, and small intermediate metabolites are carried into the brain via facilitated transport, whereas larger molecules such as insulin and 1172 transferrin are carried across the endothelial layer via receptormediated endocytosis. When compared with the normal, ordered vasculature of healthy tissues, blood vessels in tumors are often highly abnormal, such as distended capillaries with leaky walls and sluggish flow, and thereby lead to inconsistent drug delivery. Increased bioreductive enzyme expression is an adaptive strategy for solid tumors to detoxify anticancer drugs, and the hypoxic environment further contributes to the increased bioreductive activity of tumors. Moreover, lipophilic analogues are less soluble in brain interstitial fluid, which limits their activity against tumor cells. Lipophilic ester prodrugs of the anticancer agent chlorambucil have been developed to increase efficacy in the treatment of brain tumors.

It is not unusual for a tumor of perhaps 20 g to produce a mass of nearly 100 mL because of the associated edema antimicrobial on air filters studies about cheap sumycin. Cerebral edema may also be produced by an extra-axial tumor, in which case the edema, also vasogenic in origin, comes only from the brain tissue itself antibiotics chicken buy discount sumycin 250 mg. As the tumor infiltrates along nerve fiber tracts or surrounds neurons (satellitosis), it ultimately interferes with neurological function antibiotic generations purchase discount sumycin. Seizures are a common manifestation of intracranial tumor, both generalized and focal bacteria that causes uti buy sumycin 500 mg lowest price. Finally, the tumor itself may hemorrhage, producing acute headache and focal localizing signs antibiotic resistance lab generic 500 mg sumycin free shipping. Tumors that are particularly likely to hemorrhage include glioblastomas and metastatic tumors from melanoma, choriocarcinoma, and testicular carcinomas. General manifestations include mental changes, headache, generalized convulsions, nausea, and vomiting. Examples of focal signs include focal seizures, weakness, sensory abnormalities, speech disturbances, and visual defects. It is important to recognize that general and focal manifestations overlap, as does their pathogenesis. Thus, although mental dullness often accompanies large masses, mental changes may occur with small focal tumors in the temporal lobe, or may be part of an aphasia syndrome. In addition to its size, the location of the tumor in the brain quantitatively influences signs and symptoms. Thus, a small tumor in the speech or visual association cortex may produce more signs than does a large frontal tumor. In addition, the character of an abnormality may differ according to whether it accompanies a generalized increase in intracranial pressure or is part of a specific focal dysfunction. Psychomotor retardation is the most common mental change accompanying a brain tumor. Characteristically, impersistence in routine tasks, emotional lability, inertia, faulty insight and forgetfulness, reduction in the range of mental activity, indifference to social practices, reduced initiative and spontaneity, and blunted affect are seen. Patients may sleep for longer periods, complaining that they seem unable to get through a day without taking a nap. Frank confusional states and dementia usually occur later and are more likely to accompany focal signs of brain disease. Alternatively, the change in the state of consciousness may progress to stupor and frank coma. Changes in personality may be described in "psychological terms": depression, euphoria, impulsive behavior. Mental changes must be viewed together with other signs and symptoms, and especially with a history of progression of symptoms, to lead the physician to suspect a neoplastic process. It is often aggravated by the Valsalva maneuver, as in coughing or straining, and frequently becomes worse through change of posture. The headache that occurs in the early morning hours appears to be related to the recumbent posture and is thought to be associated with increased intracranial pressure as a result of lying down. Headache Headache occurs from traction of basal meningeal structures and is worse the larger the mass, although even small tumors may produce such traction, especially meningiomas. The degree and intensity of headache also relate to the rapidity with which traction develops. A rapidly enlarging intracranial mass may produce sudden severe headache, whereas a slowly growing mass may become quite large without producing headache. Headache occurs in about 50% of patients with brain tumor at some time during the course of the illness. Of special importance is a Generalized Convulsions Seizures occurring for the first time in adults are more likely to be due to focal cerebral disease, especially neoplasms, than are seizures that begin in childhood, which are more often part of the epilepsies that are characteristic of this age group. Petit mal epilepsy is probably never due to a neoplasm, but minor temporal lobe seizures (partial simple or complex) that resemble petit mal attacks may accompany temporal lobe tumors. Jacksonian seizures that progress from one body part to another usually imply a lesion of the motor or sensory cortex. Seizures, both generalized and focal, occur in 25% to 50% of patients with cerebral tumors (Table 108-3). Seizures have been reported to occur in 25% of patients with reported malignant gliomas. A syndrome of low-grade gliomas as a cause of chronic epilepsy has been recognized. Partial complex seizures are much more frequent with tumors of the temporal lobe than tumors located elsewhere in the brain. Seizures infrequently accompany infratentorial childhood brain tumors but occur in 22% of children younger than 14 years with supratentorial tumors and 68% of older teenagers with such tumors. In the older literature, papilledema was a frequent finding in patients with brain tumors. Thus, Huber15 reported that of his 1166 patients with brain tumor, 59% had papilledema, and 41% did not. In recent years, the overall incidence of papilledema in patients with brain tumor has decreased; currently, fewer than 20% of such patients have papilledema, although it is more common with increased intracranial pressure. A jacksonian seizure is most likely to occur with central-parietal tumors, whereas partial complex (psychomotor) seizures are usually associated with temporal lobe tumors. Alteration of vision implies involvement of the visual apparatus, including a problem in the eye itself, the occipital cortex, or the oculomotor nerves. Visual loss may involve reduced visual acuity, the occurrence of field defects, or diplopia. Parietal lesions and temporal lobe lesions produce corresponding, variably congruent homonymous quadrantanopia. Pupillary abnormalities and oculomotor abnormalities may be caused by tumors growing in the base of the brain and involving the third cranial nerve, fourth nerve, or sixth nerve. As noted previously, a sixth nerve paresis may accompany mass lesions as a false localizing sign. Hearing impairment may mean reduced auditory acuity or the occurrence of tinnitus and may be associated with vertigo. Hearing impairment is especially common in tumors that involve the eighth cranial nerve but may also accompany meningeal carcinomatosis and tumors of the fifth cranial nerve. Speech disturbances include impaired spoken language and understanding speech and may be transient or progressive; expressive and receptive dysphasia progressing to aphasia occurs. Generally, tumors involving the dominant posterior-inferior frontal lobe produce an expressive aphasia; those involving posterior parietal or posterior temporal lobe produce mixed receptive-expressive aphasia. A speech disorder involving articulation, called dysarthria, can accompany tumors of the posterior fossa or cerebral hemisphere and is not unusual in meningeal carcinomatosis. Subcortical tumors of the hemispheres frequently produce weakness of the contralateral body side. Usually increased deep tendon reflexes and other evidence of increased tone are seen. Posterior fossa tumors may produce long-tract signs with accompanying weakness and incoordination. Cerebellar tumors frequently produce incoordination, difficulty in walking, and dysmetria (uncoordinated rapid alternating movements or point-to-point testing. Sensory disturbances include paresthesias (tingling sensations), numbness, or altered sensation. Cortical (usually parietal lobe) disturbances include proprioception loss, sensory discrimination (two-point, graphesthesia), and astereognosis. Gait disorders may accompany weakness, incoordination, or sensory disturbances and include falling and ataxia. Ataxia may accompany tumors in the posterior fossa or, occasionally, in the frontal lobes (apractic gait). Anosmia may be associated with infrafrontal meningiomas, which can compress the olfactory or first cranial nerve. As noted previously, cerebral hemorrhage into a tumor (usually choriocarcinoma), testicular tumors, melanomas, glioblastomas, and, less frequently, other tumors may also be associated with subarachnoid hemorrhage. Skull tumors, especially metastatic, may be associated with subdural collections of tumor and subdural effusions; the patient may present as if a chronic subdural hematoma were present. Meningeal carcinomatosis is frequently associated with multifocal neurological signs and symptoms, including cerebral, cranial nerve, and spinal cord and nerve disturbances. Tumors of the cerebral hemispheres are characterized by progressive, focal neurological deficit, and commonly by generalized or focal convulsive seizures. In addition, all motor function requires sensory input, and extensive connections link the frontal lobes with the somesthetic regions of the parietal areas and thalamus, along with visual input from the occipital lobes and auditory input from the temporal lobes. The frontal lobes also have widespread connections to limbic areas of brain, through which emotional tone is controlled. The disorders associated with frontal lobe lesions include intellectual impairment, impairment of initiative and spontaneity, personality changes, and motor disturbances. The disturbances may be sufficiently serious that they may predict survival after treatment. If the dominant frontal lobe is involved, aphasia is common; this is generally expressive in type but can be mixed receptive and expressive. Impairment of Initiative and Spontaneity A common manifestation of intellectual impairment is the inability to initiate tasks. Patients lose interest in their surroundings, often staring blankly when asked to become involved in family or business activity. They remain in bed and may not dress, preferring to sit around the house, often wandering aimlessly or watching television without much involvement in the programs. When severe, the patient has the characteristics of akinetic mutism; although paralysis is not seen, immobility may last for days. By contrast, the patient may be irritated by being disturbed and may respond angrily and inappropriately. Personality Changes Two types of personality changes may accompany a frontal lobe lesion: apathetic and indifferent (pseudodepressed) and euphoric (pseudopsychopathic). Pseudopsychopathic patients vary from those whose humor becomes inappropriate, such as making silly jokes (witzelsucht), to those who exhibit socially unacceptable behavior, such as disrobing or urinating in public. Although these dramatic examples do occur, by far the most common personality change is one of disinterest, inattentiveness, impersistence, and sometimes drowsiness. Motor Disturbances Patients with frontal lobe tumors almost always develop motor problems. A contralateral hemiparesis is common, usually associated with hyperreflexia and an extensor plantar response (Babinski). Butterfly gliomas may be associated with a magnetic-like apractic gait in which balance is disturbed and the patient has difficulty initiating walking. A tumor of the medial surfaces of the frontal lobes may cause precipitate urination. Frontal Lobe Tumor the frontal lobes are thought to contain the "seat of the intellect," but the brain works as an integrated whole and requires extensive interneuronal connections among multiple regions for its cognitive functions. Seizures from lesions in the hippocampal gyrus may include odd, often unpleasant odors as part of the aura-so-called uncinate fits. Tumors involving the surface of the dominant temporal lobe produce mixed expressive and receptive aphasia or dysphasia, chiefly anomia. Cerebellopontine Angle Tumor Cerebellopontine angle tumors, particularly acoustic or vestibular schwannomas, impair function of the eighth cranial nerve and are characterized by unilateral hearing impairment, tinnitus, and sometimes vertigo. Pressure on the adjacent cranial nerves, brainstem, and cerebellum produces loss of corneal reflex, facial palsy and anesthesia, palatal weakness, signs of cerebellar dysfunction, and, rarely, contralateral hemiplegia or anesthesia. Parietal Lobe Tumor Parietal lobe tumors may provoke either generalized convulsions or sensory focal seizures. Cutaneous tactile, pain, and temperature senses are intact, but stereognosis and the cortical sensory modalities (position sense, two-point discrimination) are impaired on the contralateral body side. Contralateral homonymous hemianopia (or inferior quadrantanopia), apraxia, and anosognosia (nonrecognition of bodily defects) may also be present. Speech disturbances, notably receptive aphasia or mixed expressive-receptive aphasia, agraphia, and finger agnosia, may occur when the tumor involves the dominant hemisphere. Pituitary and Suprasellar Tumor Pituitary and suprasellar tumors produce neurological and endocrinologic abnormalities. Pituitary adenomas may present as intrasellar secretory or nonsecretory masses, or masses with extrasellar extension. Enlarging pituitary adenomas cause headache; as the tumor grows out of the sella, it compresses the optic chiasm, nerve, or tracts, as well as the hypothalamus. The most common visual field defect is bitemporal hemianopia, but unilateral optic atrophy, contralateral hemianopia, or any combination of the three may occur. Hypothalamic compression usually causes diabetes insipidus from injury to the supraoptic-pituitary tract. The tumor may destroy functioning glandular tissue and cause pituitary deficiency. Occasionally, acute degeneration (hemorrhage or infarction) of a pituitary tumor produces pituitary apoplexy, the syndrome of sudden headache, amblyopia, diplopia, drowsiness, confusion, or coma. Associated convulsions may be preceded by an aura of flashing lights but not usually formed images. Subcortical Tumor Subcortical tumors commonly involve the internal capsule and produce contralateral hemiparesis or hemiplegia. They may invade any of the lobes of the hemisphere, producing corresponding symptoms. Thalamic invasion produces contralateral cutaneous sensory impairment and occasionally abnormal eye movements. Invasion of the basal ganglia usually does not produce parkinsonian symptoms, but athetosis, bizarre tremors, or dystonic postures occasionally occur.
Classification of brain tumors is an evolving process, with obsolete entities being discarded and newly recognized tumors added with each successive revision antibiotics for uti co amoxiclav buy 500 mg sumycin mastercard. In the past, classification has relied heavily on recognition of morphologic patterns and immunohistochemical identification of differentiation antigens, but with the discovery a decade ago of the association between the translocation and subsequent deletion of chromosomal arms 1p and 19q and the responsiveness of anaplastic oligodendroglioma to treatment, a new era of molecular classification of brain tumors began bacteria notes cheap sumycin on line. Current advances in molecular methodologies, particularly in the fields of genomics, transcriptonomics, and proteomics, have revolutionized brain tumor classification, and although the present classification remains based on morphology, histology is increasingly being complemented by genetic characterization of neoplasms infection elite cme com continuing education order sumycin 250mg with mastercard. Complete surgical resection is rarely attained with diffuse astrocytoma because of the inherently infiltrative nature of the neoplastic cells, which extend well beyond the apparent gross margin of the tumor infection in bone order sumycin on line amex. Mitotic figures are either absent or rare, and there is no vascular proliferation or necrosis virus vs infection purchase sumycin 500mg otc. Invasion of the overlying leptomeninges is common, but this feature does not constitute a negative prognostic factor. Microscopically, many pilocytic astrocytomas exhibit a biphasic architectural pattern consisting of compacted areas of elongated, piloid (hair-like) cells alternating with loosely textured and microcystic areas populated by scattered stellate cells. In pilocytic astrocytomas, mitotic activity, vascular proliferation, and necrotic foci do not have the same ominous prognostic significance as when present in diffuse astrocytomas. In favorable anatomic locations, such as the cerebellum, surgical resection of pilocytic astrocytoma has the potential to be curative. Distinctive histologic features include a monomorphic population of neoplastic pilocytes in a prominent myxoid background stroma. The pleomorphic, giant, and often multinucleated cells may display a variable xanthomatous change in their cytoplasm because of intracellular accumulation of lipids. As with pilocytic astrocytoma, invasion of the overlying meninges is characteristic and does not constitute a negative prognostic factor. This tumor is almost invariably associated with tuberous sclerosis, although this condition is often not known at initial evaluation. Both lesions share a superficial cerebral cortical location, large size, circumscribed growth pattern, and development during infancy. Vascular proliferation is defined as the presence of blood vessels with multilayered vessel walls (more than two cell layers thick). In contrast to diffuse astrocytomas, pilocytic astrocytomas exhibit very little tendency for anaplastic progression. Surgical resection is the treatment of choice, and the prognosis is generally more favorable than would otherwise be suggested by the typically very large size of most of these tumors at diagnosis. Their preferential location is the white matter of the cerebral hemispheres, from which tumor cells typically infiltrate the overlying cortex. As viewed macroscopically and on neuroimaging studies, oligodendrogliomas often appear somewhat more circumscribed than astrocytomas. They are composed of uniform round cells with cleared cytoplasm surrounding a central spherical nucleus (fried egg appearance). The perinuclear halo is a diagnostically useful fixation artifact present only in formalin-fixed, paraffin-embedded tumor tissue. A branching network of small delicate blood vessels (chicken wire pattern) is a classic histologic feature of many oligodendrogliomas. Subpial tumor infiltration, perineuronal satellitosis, and perivascular satellitosis of tumor cells (secondary structures of Scherer) are characteristically seen in oligodendrogliomas that infiltrate gray matter. No oligodendroglioma-specific immunohistochemical markers are currently available. Oligodendrogliomas generally recur locally and ultimately undergo anaplastic progression. The hallmark genetic signature of oligodendroglioma (low grade and anaplastic) is combined whole-arm deletion of chromosomes 1p and 19q, which arises secondary to an initial translocation event and constitutes an independent prognostic marker, with 1p or 19q loss being associated with improved outcome regardless of the specific therapeutic regimen. There is no consensus on the minimal percentage of each component required for diagnosis of a mixed glioma. In general, anaplastic oligoastrocytoma exhibits high-grade features such as increased mitotic activity and often microvascular proliferation, but otherwise it has the same subjective diagnostic criteria as oligoastrocytoma. Anaplastic oligoastrocytomas that exhibit deletion of both 1p and 19q are considered to have a favorable genetic signature, whereas those with intact 1p/19q status and p53 immunopositivity are considered genetically closer to astrocytomas. An infratentorial location is the most frequent in children, whereas in adults most of these tumors are supratentorial. Ependymomas may occur outside the ventricular system in the brain parenchyma and also in the spinal cord. As seen histologically, classic ependymomas are moderately cellular tumors composed of oval cells with monomorphic nuclei and tapering eosinophilic cytoplasm. Some ependymomas have a more glial appearance, whereas others are more epithelioid. Some ependymoma variants (cellular, tanycytic) mimic other primary tumors, although others (papillary, clear cell) may mimic secondary tumors. The histologic hallmarks of ependymoma are the perivascular pseudorosette (perivascular collars of radiating tumor cell cytoplasmic processes) and, more elegantly but less frequent, the true ependymal rosette (tumor cells surrounding a central lumen). Electron microscopy may be required in some cases to identify the ultrastructural features associated with ependymal cell differentiation (intercellular lumina filled with microvilli and sometimes cilia and prominent intercellular junctional complexes). The tumor is usually well circumscribed and covered by an outer layer of investing leptomeninges (capsule). Surgical resection of intact tumors can be curative in some cases; however, the presence of micrometastases, which are not visible to the unaided eye, or frank capsular breaching by the tumor can lead to diffuse dissemination among the nerve roots of the cauda equina and ultimately result in significant morbidity. Curative surgery is still possible, but the probability of recurrence appears to be significantly higher. The choroid plexus may also be involved by a variety of other neoplastic and nonneoplastic mass lesions, most prominently intraventricular meningioma, metastatic carcinoma (especially renal cell carcinoma), and xanthogranuloma (which is a reactive mass lesion characterized by cholesterol clefts and attendant multinucleated giant cell reaction). Chordoid glioma is hypothesized to originate in association with the specialized ependymal cells of the lamina terminalis circumventricular organ (organum vasculosum of the lamina terminalis). The tumor is well circumscribed, but its anatomic origin, close to the hypothalamic region, often precludes total resection. The histogenesis of angiocentric glioma is uncertain, with the differentiation features overlapping those of both astrocytic and ependymal tumors. Astroblastoma is a rare glioma, viewed as a subtype of ependymoma by some, that primarily arises in children and young adults. Areas with prominent vascular mural thickening and hyalinization are very characteristic. Patients with low-grade tumors have a good prognosis after undergoing gross-total resection. Asymptomatic lesions are often discovered only as incidental findings at autopsy, but subependymomas occasionally produce ventricular obstruction of the lateral or fourth ventricles. As seen microscopically, the tumor is composed of clusters of small, uniform, benign-appearing tumor cell nuclei separated by extensive cell-free areas of finely fibrillary matrix. Lateral ventricular examples are prone to prominent microcystic change that can obscure the characteristic multinodular architecture. As viewed both macroscopically and microscopically, choroid plexus papilloma closely recapitulates the papillary architecture of normal choroid plexus, but the tumor cells tend to be more crowded and columnar, as opposed to the cuboidal morphology of normal choroid plexus epithelium. Some cases of choroid plexus carcinoma require ultrastructural identification of the characteristic features of choroid plexus differentiation (microvilli, cilia, and intercellular junctional complexes). Ganglioglioma is the most common tumor associated with chronic temporal lobe epilepsy (40% of tumor-associated temporal lobe epilepsy cases). Both tumors are grossly circumscribed, may be solid or cystic, and frequently contain calcifications. At the microscopic level, gangliocytoma is composed entirely of clusters of dysmorphic mature ganglion cells. Gangliocytomas are benign tumors composed exclusively of mature "ganglion cells" that exhibit the cytologic features of large neurons, including prominent single nucleoli and cytoplasmic basophilic Nissl substance. In the latter two cases, ganglioglioma has the potential to undergo anaplastic progression. Ganglioglioma must be differentiated from the cortical invasion of diffuse astrocytoma with entrapped neurons. Immunopositivity for neuronal markers, such as synaptophysin and NeuN, in a subpopulation of tumor cells is characteristic. A layer of myelinated axons in the outermost part of the molecular layer just beneath the pia is also a distinctive feature. The rostral septum pellucidum/head of the caudate nucleus/frontal horn of the lateral ventricle region is an additional rare but well-recognized site of occurrence. Resection is curative, and even partial resection usually stops the seizure activity. Neurocytomas are typically located in the lateral ventricles or third ventricle, or both, with an attachment to the septum pellucidum. Neoplasms with similar histopathologic characteristics and biologic behavior occur outside the ventricular system. Surgery can be curative with small lesions, but local recurrence results with partially resected tumors. Increased mitotic activity and vascular proliferation may rarely be seen but are not generally associated with a poor prognosis. As seen histologically, paragangliomas of the filum terminale can mimic ependymoma, with perivascular pseudorosette formation. Most tumors are encapsulated by an investing layer of leptomeninges and may be cured by total excision. Papillary glioneuronal tumor is a supratentorial lesion (often temporal) histologically characterized by pseudopapillary structures of cuboidal glial cells surrounding hyalinized vessels, with the intervening zones filled with neurocytic elements. Rosette-forming glioneuronal tumor of the fourth ventricle is a tumor of children and young adults. The neuronal component consists of neurocytes that form rosettes with eosinophilic, synaptophysin-positive avascular cores, and the glial component typically exhibits features of pilocytic astrocytoma. Both papillary glioneuronal tumor and rosette-forming glioneuronal tumor of the fourth ventricle are clinically indolent and surgically curable. The tumor is composed of well-differentiated, uniform, matureappearing pineocytes arranged in lobules and often forming large rosettes with solid fibrillar cores (pineocytomatous rosettes). Approximately 20% of pineal parenchymal tumors exhibit higher cellularity, nuclear atypia, occasional mitoses, and absence of pineocytomatous rosettes. Flexner-Wintersteiner rosettes (small rosettes with a central lumen) or fleurettes, which are indicative of retinoblastic differentiation, may be seen. Pineoblastomas are immunoreactive for neuronal markers such as synaptophysin and retinal S-antigen. As viewed ultrastructurally, tumor cells exhibit features of ependymal differentiation. Local recurrence is common, but because of the rarity of the lesion, the biologic behavior and histologic grading of papillary tumor of the pineal region remain to be defined. Germinoma characteristically exhibits a biphasic cell population, very large malignant cells resembling primitive germ cells and small reactive lymphocytes. A prominent granulomatous response is occasionally present and, in such cases, can overshadow the tumor cell component. In some cases, -human chorionic gonadotropin immunostaining identifies the presence of isolated syncytiotrophoblastic cells. Any positive marker may be clinically useful for monitoring response to treatment and tumor recurrence through measurement of the specific marker or markers in serum. Patients may be treated with radiation therapy, chemotherapy, or a combination of both. Accurate histologic subtyping of these tumors is critical for planning treatment and predicting outcome. After germinoma, teratoma is the most common member of this group to occur as a pure (nonmixed) tumor. The prognosis of nongerminomatous germ cell tumors is generally poorer than that for germinoma and largely dependent on the extent of surgical resection. The tumor develops primarily in children, with a peak incidence at 7 years, but there is a second incidence peak in adulthood in the 21- to 40-year-old age group. Neuroblastic (Homer-Wright) rosettes are typical, but they are present in no more than 40% of cases. As seen microscopically, this variant is characterized by large lobules of neuropil-like tissue with prominent streaming of neoplastic neurocytes and little or no extranodular tissue. In contrast, the large cell variant is composed of a more monomorphous population of large cells with round vesicular nuclei and prominent nucleoli. Medulloepithelioma is the designation reserved for tumors that recapitulate the features of the primitive neural tube. Atypical teratoid/rhabdoid tumor is a rare neoplasm of childhood characterized by a unique combination of rhabdoid and primitive neuroectodermal cells, and it can show divergent differentiation along epithelial, mesenchymal, neuronal, or glial lines (or any combination of such lines). The posterior fossa is the most frequent location for atypical teratoid/ rhabdoid tumor (75% of cases), followed by the supratentorial compartment (25% of cases). The meninges covering the cerebral convexities, the falx cerebri, and the skull base are the most frequent sites of origin. Meningiomas may originate in any location where arachnoidal cells are present, including the choroid plexus (intraventricular meningioma). Meningiomas are common tumors and account for an estimated 13% to 26% of primary intracranial neoplasms. Most meningiomas are well-defined, lobulated, firm masses that compress the underlying brain. Invasion of brain parenchyma is associated with a greater likelihood of recurrence. There are nine benign morphologic subtypes that have no associated prognostic significance and four variants that are characterized by more aggressive clinical behavior. The meningothelial, transitional, and fibrous subtypes are most common in low-grade meningioma.
However, for most neoplasms, survival has not yet been shown to be superior after intrathecal treatment treatment for uti breastfeeding order discount sumycin line. Additional investigations are needed to further define the optimal timing and route of administration of drugs and radiotherapy, identify those at high risk, ascertain any role for preventive therapy, and explore new drug strategies antibiotics for acne inversa purchase sumycin 250 mg line. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy, and symptom management antibiotics for acne singapore cheap 500mg sumycin with visa. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomized study bacteria and viruses worksheets cheap sumycin 500mg with mastercard. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis yeast infection 8 weeks pregnant trusted sumycin 250 mg. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously treated neoplastic meningitis. Although there were some first descriptions of ventricular neoplasms by Shaw in 1854, it was Walter Dandy who had the most impact on descriptions of and treatment options for ventricular pathologies. During his career, he published more than 30 articles on these tumors and pioneered several surgical approaches to them that are still used today. In 1933, he dedicated a book to this subject, Benign Tumors in the Third Ventricle of the Brain: Diagnosis and Treatment. As a result, many studies and books were published on this topic in the following years, including milestones such as Surgery of the 3rd Ventricle by Michael L. We focus on surgical indications and various operative approaches and corridors to the ventricular system and discuss tips and pitfalls of different surgical methods. The preoperative and postoperative medical management of patients with these tumors is highlighted, as well as the introduction of adjuvant therapies. Secondary tumors include meningiomas, gliomas, pituitary adenomas, and arachnoid cysts. Table 138-1 lists the variety of pathologic lesions encountered at our institution in a series of 143 patients who were systematically monitored after surgery. Because this additional patient group has not yet been monitored systematically in the same fashion as the population just mentioned, only a few cases from this second patient group have been included in this chapter. We have divided the lesions in our follow-up series into five groups according to tumor location: 22% of tumors were located within both lateral ventricles, 18. Overall, tumors varied from a few millimeters to 60 mm in maximum diameter, but in 40% of patients, the range was 11 to 20 mm. Because many ventricular tumors are benign and slow growing, they may reach considerable size before causing nonspecific symptoms. In some instances, special neurological symptoms such as hemianopia, hemiparesis, and hemihypoesthesia can develop. For instance, craniopharyngiomas may cause endocrine dysfunction with stunted growth, hypothyroidism, and hypogonadism. In adults, visual disturbances (caused by compression of the chiasm) and mental disturbances can frequently be observed. According to Casotto and coworkers,5 these lesions can be categorized as primary or secondary. Primary, or true, ventricular tumors are those originating from the ventricular wall and extending strictly into the ventricular system. Paraventricular, or secondary, tumors are those originating from structures adjacent to the ventricular system. The major part of such neoplasms is located within the ventricular cavity, but a portion of the tumor is outside the ventricular system. This syndrome is caused by compression of the tectal region of the midbrain by tumors of the pineal region and tectal plate. Tumors of the fourth ventricle, such as medulloblastomas, most frequently cause cerebellar ataxia. In our series, the duration of symptoms from onset to therapy varied from a few hours to 15 years. In almost half the study population, the duration of symptoms ranged from 2 to 8 weeks. The structure is typically homogeneous, with the presence of calcifications being an exception. Intratumoral cysts may be apparent, and approximately half of the tumors show calcifications. Frequently, they are seen as hypointense or isointense lesions with cystic portions. Intratumorally, necrotic areas, blood vessels, hemorrhage, or hemosiderin may be present. Contrast enhancement is high, and calcifications or bleeding can frequently be observed. Low-grade astrocytomas appear as hypodense structures that show calcification in up to 15% of cases. Anaplastic astrocytomas are heterogeneously contrastenhancing lesions that only rarely show calcifications. Glioblastomas typically show irregular contrast enhancement on the periphery with necrotic areas in the center. It is divided into the anterior (frontal) horn, the body (cella media), the atrium (trigone), and the occipital and temporal horns. Both lateral ventricles communicate with the third ventricle via the foramina of Monro and are surrounded by the following structures: septum pellucidum, thalamus, caudate nucleus, corpus callosum, and fornix. The largest structure in connection with the lateral ventricle is the corpus callosum. The roof is formed medially by the stria terminalis and laterally by the tail of the caudate nucleus and tapetum. On the medial side above the hippocampus are the fimbria fornicis and the choroidal fissure. The choroidal fissure, to which the choroidal plexus is attached, is a small cleft between the thalamus and fornix. It extends from the foramen of Monro backward along the body, atrium, and temporal horn of the lateral ventricle. The arterial supply of the choroid plexus is derived from the anterior and posterior choroidal arteries through the choroidal fissure. Important veins are the anterior septal and caudate veins, the thalamostriate vein, the superior choroidal vein, and the medial atrial vein, which drain into the internal cerebral vein. The inferior choroidal vein, the inferior ventricular vein, the veins of the amygdala and hippocampus, and the lateral atrial vein drain into the basal vein of Rosenthal. Tumors of the lateral ventricle can originate from the periventricular white matter extending into the ventricle, from structures within the ventricle (such as the ependyma or the choroid plexus), or from ectopic tissue that has been trapped within the ventricle. The tumors commonly found here are astrocytomas, ependymomas, choroid plexus papillomas, neurocytomas, tuberous sclerosis tumors, and meningiomas. Their vascular supply varies according to the exact location, but their main feeder vessels are the anterior choroidal and posterior lateral choroidal arteries. The caudate nucleus, a part of the corpus striatum, is divided into a head, body, and tail. It covers the lateral border of the anterior horn and the body of the lateral ventricle and is part of the roof of the temporal horn. In close association, the stria terminalis runs between the caudate nucleus and thalamus and from the temporal horn to the body. The fornix connects the hippocampus with the hypothalamus and various other structures. It originates in the alveus and runs as the fimbria along the medial aspect of the hippocampus on the floor of the temporal horn. Then it arches as the crus around the thalamus under the splenium and proceeds rostrally over the thalamus to join the crus on the opposite side, where it forms the body of the fornix lying beneath the corpus callosum. At the level of the foramen of Monro they separate again, curve ventrally around the foramen to form the columns that run posterior to the anterior commissure and into the hypothalamus, and finally reach the mamillary bodies. The frontal horn is the part of the lateral ventricle lying anterior to the foramen of Monro. The medial wall is formed by the septum pellucidum and the lateral wall, by the head of the caudate nucleus. The roof and floor of the anterior horn are formed by the genu and rostrum of the corpus callosum. The body of the lateral ventricle extends from the foramen of Monro backward to the junction of the corpus callosum, with the fornix located at the posterior end of the septum pellucidum. The lateral wall is formed by the body of the caudate nucleus, the medial wall by the septum pellucidum and the fornix, the roof by the body of the corpus callosum, and the floor by the thalamus. The atrium lies between the body of the lateral ventricle and the temporal and occipital horns. The medial, superior, and lateral walls are formed by fibers of the corpus callosum, called the forceps major and tapetum. The tapetum itself is part of the sagittal stratum that also contains the optic radiation. The floor is formed by the collateral eminence (trigone) and the calcar avis, which represent bulges over the posterior end of the collateral sulcus and the calcarine sulcus, respectively. As in the atrium, the roof and the lateral wall are formed by commissural fibers of the corpus callosum (tapetum). The floor is formed by ThirdVentricle the third ventricle is located in the middle of the brain; it is connected to the lateral ventricles by the foramina of Monro and to the fourth ventricle by the aqueduct of Sylvius. The lateral walls are formed by the thalamus and hypothalamus and separated by the hypothalamic sulcus. In approximately 75% of patients, a connection between the lateral walls can be found in the upper part of the third ventricle and is referred to as the massa intermedia. The roof of the third ventricle extends from the foramen of Monro to the suprapineal recess and is formed by five layers: the fornix (superior layer), the two layers of the tela choroidea (which envelope the vascular layer [velum interpositum]), and the choroid plexus layer. The vascular layer contains the internal cerebral veins and the medial posterior choroidal arteries. In 30% of cases, this junction is located 3 to 7 mm behind the posterior border of the foramen of Monro. The two internal cerebral veins run close to each other up to the pineal recess, where they deviate from the midline and proceed along the superolateral surface of the pineal body to the deepest point of the splenium to form the vein of Galen. The plexus of the third ventricle is attached to the lower layer of the tela choroidea. The anterior border of the third ventricle extends from the optic chiasm to the foramen of Monro and is formed by the optic chiasm, the lamina terminalis, the anterior commissure, and the column of the fornix. The posterior border of the third ventricle extends from the aqueduct of Sylvius to the suprapineal recess. Between these structures are the posterior commissure, the pineal body (with its recess), and the habenular commissure. Anatomically, tumors of the third ventricle can originate from three different regions: (1) from the periventricular, mainly sellar or suprasellar region, with expansion into the ventricle. Its course can be divided into five segments: the anterior medullary segment, the lateral medullary segment, the tonsillomedullary or posterior medullary segment, the telovelotonsillar or supratonsillar segment, and the distal segment. Primarily, the first three segments are the origin of the arterial supply to the lower brainstem and vermis, but these segments may also supply tumors located in the fourth ventricle. It is also important to analyze the vascular supply of the tela choroidea and choroid plexus because tumor-supplying branches may also emerge from these vessels. Thus, some tumors with exophytic growth may consist of a limited tumoral and vascular pedicle and a large portion that compresses the brainstem but is not completely attached to the floor of the fourth ventricle. The lateral extension of the neoplasm may be confined to the fourth ventricle or may extend beyond it. This is the case when a medulloblastoma, ependymoma, or glioma is expanding through the foramen of Luschka into the cerebellopontine cistern, where it may encase the rootlets of the caudal cranial nerves. Large tumors may also extend caudally beyond the level of the obex and fill the space dorsal to the superior cervical cord with or without invading the neuraxis. This initial procedure may be followed by microsurgical resection of the underlying ventricular tumor. In other instances, such as with tumors of the pineal region, patients may first require endoscopic or stereotactic biopsy. Although most surgical interventions for ventricular tumors are elective surgical procedures, emergency surgery may be required in patients with acutely developed obstructive hydrocephalus or when acute intratumoral hemorrhage has occurred. FourthVentricle the fourth ventricle is located between the cerebellum posteriorly, the brainstem anteriorly, and the middle cerebellar peduncles. In the craniocaudal direction, the fourth ventricle extends from the aqueduct of Sylvius to the obex. This ventricle has a ventral floor formed by the dorsal surface of the lower midbrain (the pons and medulla inferiorly); a lateral wall formed by the superior, medial, and inferior cerebellar peduncles; and the lateral recess, in which the fourth ventricle communicates with the cerebellopontine angle. The superior part of the roof of the fourth ventricle is formed by the lingula, the superior medullary velum, and the fastigium, whereas the inferior part of the roof is formed by the tela choroidea, the choroid plexus, the inferior medullary velum, and the uvula and nodulus of the vermis. To obtain a wide view into the fourth ventricle up to the aqueduct, the lower vermis must be elevated and retracted dorsally and superiorly. For this purpose, arachnoid dissection and sectioning of the tela choroidea are necessary. In practice, the so-called telovelar approach provides the best access inferiorly to the fourth ventricle. Lesions located within the fourth ventricle originate either from the part of the brainstem that forms the floor of the fourth ventricle, from the tela choroidea and choroid plexus, or from various parts of the cerebellum. Patients with tumors that have caused significant perilesional edema should first be treated with high-dose steroids for 1 or 2 days. Although surgery for most tumors of the lateral and anterior third ventricle can be performed with the patient placed in the supine or lateral position, tumors of the dorsal third ventricle and those located within the fourth ventricle are often removed more safely with the patient in the sitting position. For the latter patient group, it is advisable to exclude the presence of an open foramen ovale before surgery by performing a cardiac ultrasound examination. Likewise, an endoscope should be prepared for use during the microsurgical procedure as an assisting tool because it can easily visualize hidden corners of the ventricular system during the procedure. If indicated and possible, endoscopic third ventriculostomy can be applied for the same reason.
Buy sumycin 500mg with amex. Blood cancer experiment with garlic vs Gold.
References
- Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44(2):96-104.
- Furyk JS, Chu K, Banks C, et al: Distal ureteric stones and tamsulosin: a double-blind, placebo-controlled, randomized, multicenter trial, Ann Emerg Med 67:86n95, 2016.
- Parker C, Nilsson S, Heinrich D, et al: Alpha emitter radium-223 and survival in metastatic prostate cancer, N Engl J Med 369:213n223, 2013. Parker C, Pascoe S, Chodacki A, et al: A randomized, double-blind, dosefinding, multicenter, phase 2 study of radium chloride (Ra-223) in patients with bone metastases and castration-resistant prostate cancer, Eur Urol 63:189n197, 2013. Patchell RA, Tibbs PA, Regine WF, et al: Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial, Lancet 366:643n648, 2005.
- Brant, L.A., Brant, W.O., Brown, M.H. et al. A new minimally invasive open pelvic lymphadenectomy surgical technique for the staging of prostate cancer. Urology 1996; 47:416-421.
- Treacy EP, Lambert DM, Barnes R, et al. Short-chain hydroxyacylcoenzyme A dehydrogenase deficiency presenting as unexpected infant death: a family history. J Pediatr 2000;137:257.