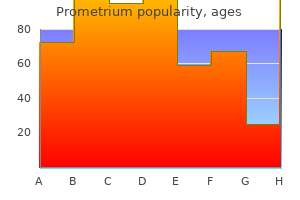
"Prometrium 200 mg free shipping, the treatment 2014".
Q. Merdarion, M.B. B.CH., M.B.B.Ch., Ph.D.
Deputy Director, University of Oklahoma School of Community Medicine
In cancer patients the drug of choice is dexamethasone medications for bipolar disorder prometrium 200 mg on-line, which provides only glucocorticoid properties symptoms 2016 flu prometrium 100mg sale, causing less fluid retention and potassium loss as compared to hydrocortisone or prednisolone medications that cause dry mouth buy discount prometrium 100 mg. There is no evidence-based dosing scheme treatment hyperthyroidism 200mg prometrium free shipping, but in acute pain exacerbation because of massive cancer progression, a common approach would be to use a loading dose of approximately 24 mg the first day and then reduce the dose subsequently over the following days to a maintenance dose of 2 mg daily. Side effects can prove to be beneficial for the patient, such as euphoria and an increased appetite in cachectic patients. Other typical side effects such as osteoporosis, skin thinning, diabetes, and adrenal suppression are of less importance in the target patient with limited life expectancy. Neuroleptics Neuroleptics are psychoactive drugs that are commonly used to treat psychotic episodes and nausea. Do not underestimate the distress for the patient and family in the presence of delirium. Most of the time it is the first sign of infection, renal failure, dehydration, or electrolyte imbalances. In rare instances, it may also be a side effect of opioid therapy (in which case, opioid rotation will solve Benzodiazepines Benzodiazepines are a group of drugs with varying sedative, anxiolytic, anticonvulsant, and muscle relaxant properties. The main indication for these drugs in pain management and the palliative care management is the treatment of anxiety and intractable dyspnea. Do not hesitate to prescribe these drugs for terminal 358 ill patients, who suffer from panic attacks, dyspnea and insomnia. The anticonvulsant properties of benzodiazepines may be in part or entirely due to binding to voltage-dependent sodium channels. If you want to treat panic attacks, use benzodiazepines with shorter half-lives, such as lorazepam. Diazepam can be administered orally, intravenously, intramuscularly, or as a suppository. Sometimes it is necessary to increase the dose extensively without negative consequences. Diazepam, in combination with morphine, is the drug of first choice for palliative sedation. For trait anxiety in terminal illness, flunitrazepam subcutaneously once daily is a very effective choice (normally in a dose range between 0. During the course of therapy with benzodiazepines, tolerance to the sedative effects usually develops, but not to the anxiolytic effects. There is no real contraindication in the palliative setting if used with care, titrated to effect, and used where indicated. Following that review, these experts agreed to take advantage of the publication of the draft collection of syndromes and their system for classification, to issue an updated list of terms with definitions and notes on usage. That need not be a cause of distress, provided that each author makes it clear precisely how he is using a word. Nevertheless, it is convenient and helpful to others if words can be used that have agreed technical meanings. The definitions provided in this Appendix are intended to be specific and explanatory and to serve as an operational framework, not as a constraint on future development. They represent agreement among diverse specialties including anesthesiology, dentistry, neurology, neurosurgery, neurophysiology, psychiatry, and psychology. The terms and definitions are not meant to provide a comprehensive glossary, but rather a minimum standard vocabulary for members of different disciplines who work in the field of pain. It is important to recognize that allodynia involves a change in the quality of a sensation, whether tactile, thermal, or of any other sort. With other cutaneous modalities, hyperesthesia is the term that corresponds to hyperalgesia, and as with hyperalgesia, the quality is not altered. Allodynia might be provoked by the touch of clothes, such as in patients with postherpetic neuralgia. Apart from coanalgesics, local treatment with local anesthetics and/or capsaicin might be of help. Acupuncture Acupuncture is a procedure involving the stimulation or inhibition of anatomical locations on or in the skin by a variety of techniques. A number of effects on pain physiology have been identified, the most important being the activation of the endogenous opioid system and the spinal modulation of pain signalling through activation of touch fibers (A fibers).
Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled treatment yeast diaper rash 200mg prometrium free shipping, type 2 diabetes: Ginseng + Alcohol Panax ginseng (Asian ginseng) increases the clearance of alcohol and lowers blood-alcohol levels medicine x xtreme pastillas purchase prometrium 100 mg free shipping. Clinical evidence Fourteen healthy subjects medicine just for cough safe prometrium 100 mg, each acting as their own control medications excessive sweating prometrium 100 mg online, were given alcohol (72 g/65 kg as a 25% solution) with and without a Panax ginseng (Asian ginseng) extract (3 g/65 kg) mixed in with it. They drank the alcohol or the alcohol/ginseng mixture over a 45minute period in 7 portions, the first four at 5-minute intervals and the next three at 10-minute intervals. Measurements taken 40 minutes later showed that the presence of the ginseng lowered bloodalcohol levels by an average of about 39%. The alcohol levels of 10 subjects were lowered by 32 to 51% by the ginseng; 3 showed reductions of 14 to 18% and one showed no changes at all. Importance and management Evidence for an interaction between Panax ginseng (Asian ginseng) and alcohol comes from a clinical study, which confirms the initial findings of some experimental studies. Importance and management these studies suggest that Panax ginseng (Asian ginseng) is unlikely to affect the metabolism of caffeine. Therefore it would not be expected to reduce the effects or increase the adverse effects of caffeine. Nevertheless, ginseng is considered to be a stimulant, and it is possible that additive stimulant effects might occur with caffeine, although there do not appear to be many data on this. However, if both substances are given, bear the possibility of increased stimulant effects in mind. For information on one study where the stimulant effects of a caffeine-containing herb appeared to be additive to those of Panax ginseng, see Ginseng + Herbal medicines; Guarana, page 223. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Similar postprandial glycemic reductions with escalation of dose and administration time of American Ginseng in type 2 diabetes. Isolation and hypoglycemic activity of eleutherans A, B, C, D, E, F, and G: glycans of Eleutherococcus senticosus roots. Ginseng + Benzodiazepines Eleutherococcus senticosus (Siberian ginseng) did not alter the pharmacokinetics of alprazolam, and Panax ginseng (Asian ginseng) did not alter midazolam metabolism. Clinical evidence A study in 12 healthy subjects found that Eleutherococcus senticosus (Siberian ginseng), 485 mg twice daily for 15 days, did not significantly affect the pharmacokinetics of a single 2-mg dose of alprazolam given with the morning dose on day 14. Importance and management these studies suggest that both Panax ginseng (Asian ginseng) and Eleutherococcus senticosus (Siberian ginseng) are unlikely to affect the metabolism of alprazolam and midazolam. Ginseng + Carbamazepine For mention that saiko-ka-ryukotsu-borei-to and sho-saiko-to (of which ginseng is one of a number of constituents) did not affect the pharmacokinetics of carbamazepine in animal studies, see Bupleurum + Carbamazepine, page 90. Ginseng + Chlorzoxazone Panax ginseng (Asian ginseng) did not alter chlorzoxazone metabolism in one study. Clinical evidence In a study in 12 healthy subjects, Panax ginseng (Asian ginseng) 500 mg three times daily for 28 days did not significantly affect the pharmacokinetics of chlorzoxazone 500 mg. Importance and management these studies suggest that Panax ginseng (Asian ginseng) is unlikely to affect the pharmacokinetics of chlorzoxazone. Chlorzoxazone is Ginseng + Caffeine Panax ginseng (Asian ginseng) did not alter caffeine metabolism in one study. Clinical evidence In a study in 12 healthy subjects Panax ginseng (Asian ginseng), 500 mg three times daily for 28 days, did not significantly affect the pharmacokinetics of caffeine 100 mg. The combination improved both attention and memory tasks, with no clear evidence for synergistic effects, except for better performance in the increased serial sevens subtractions compared with either drug alone. In this study, the ginseng extract was standardised to 4% of ginsenosides, and the guarana extract to 11 to 13% of xanthines (caffeine and theobromine), or a maximum of about 10 mg of caffeine per dose. Mechanism Both guarana and ginseng are used for their putative stimulative effects. In this study, they affected different tasks and, in combination, their effects were generally additive. The effect of guarana was not considered to be solely attributable to the caffeine content, since the dose of caffeine was low. This study provides some evidence that they do not appear to have synergistic effects, but that the combination is the sum of the different effects of the two herbs.
Currency exposures are described in more detail in "-Effects of currency fluctuations" above medicine 75 yellow generic prometrium 200 mg mastercard. However treatment 20 buy prometrium 200 mg on-line, given the inherent difficulties in estimating liabilities in this area treatment hiatal hernia discount 100 mg prometrium fast delivery, Novartis may incur additional costs beyond the amounts provided treatment 2015 buy discount prometrium 100 mg online. In our key countries, Switzerland and the United States, assessments have been agreed by the tax authorities up to 2015 in Switzerland and 2014 in the United States, respectively, with the exception of one open United States position related to the 2007 tax filing. This decrease was mainly due to the dividend in kind to effect the spinoff of Alcon Inc. This revaluation resulted from the Swiss federal tax reform enacted in May 2019 (see "Item 18. Operating and Financial Review and Prospects to them under the respective programs. Treasury shares At December 31, 2019, our holding of treasury shares amounted to 262. Approximately 118 million treasury shares were held in entities that limit their availability for use. Approximately 122 million treasury shares were held in entities that limit their availability for use. Approximately 131 million treasury shares were held in entities that limit their availability for use. This involves modeling cash flow maturity profiles based on both historical experiences and contractual expectations to project our liquidity requirements. We are not aware of any significant demands to change the level of liquidity needed to support our normal business activities. We make use of various borrowing facilities provided by several financial institutions. We also successfully issued various bonds in previous years (including 2017 and 2018), and raised funds through our commercial paper programs. For further information on these policies and these products in development, see "Item 4. Due to the risks and uncertainties involved in progressing through preclinical development and clinical trials, and the time and cost involved in obtaining regulatory approvals, among other factors, we cannot reasonably estimate the timing, completion dates and costs, or range of costs, of our drug development program, or of the development of any particular development compound (see "Item 3. In addition, for a description of the research and development process for the development of new drugs and our other products, and the regulatory process for their approval, see "Item 4. E Off-balance sheet arrangements We have no unconsolidated special purpose financing or partnership entities or other off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources, that is material to investors. The Group intends to fund the research and development; property, plant and equipment; intangible asset purchase commitments with internally generated resources, and the acquisition of business commitment through available cash and short- and long-term borrowings. The acquisition of business commitments relate to the acquisition of the Medicines Company (see "Item 18. Significant transactions -Significant Transactions entered into in 2019 and closed in January 2020") and to the pending acquisition of the Japanese business of Aspen Global Incorporated (see "Item 18. C Board practices-Corporate governance- Executive Committee" is incorporated by reference. During 2019, the committee continued to engage with shareholders and proxy advisors to gather feedback on the compensation system for the Executive Committee and our disclosures. Our recent transactions in M&A are strengthening our innovation programs and further supporting our strategy to become a leading, focused medicines company. Recently launched products, including Zolgensma, Piqray and Beovu, also contributed to our growth. For the Executive Committee, the requested maximum aggregate amount of compensation remains broadly unchanged compared to the prior year. Shareholders will also be asked to endorse this Compensation Report in an advisory vote. On behalf of Novartis and the Compensation Committee, I would like to thank you for your continued support and feedback, which we consider extremely valuable in driving improvements in our compensation systems and practices. The payout range remains at 0% to 200% of target opportunity based on achievement against performance.
These include the medical indications for which it is being developed medications not to take during pregnancy order 200mg prometrium with visa, the number of indications being pursued treatment of tuberculosis discount prometrium 200mg free shipping, whether the molecule is of a chemical or biological nature treatment e coli cheap 200 mg prometrium overnight delivery, the stage of development medications errors pictures purchase prometrium 100 mg without a prescription, and the level of evidence necessary to demonstrate clinical efficacy and safety. We focus our work in areas where we believe we can have the most impact for patients. This requires the hiring and retention of highly talented employees, a focus on fundamental disease mechanisms that are relevant across different disease areas, continuous improvement in technologies for drug discovery and potential therapies, close alliances with clinical colleagues, and the establishment of strategic external alliances. They contribute to research into disease areas such as cardiovascular and metabolic diseases, neuroscience, oncology, muscle disorders, ophthalmology, autoimmune diseases and respiratory diseases. All drug candidates go through proof-of-concept trials to enable an early assessment of the safety and efficacy of the drug while collecting basic information on pharmacokinetics and tolerability, and adhering to the guidance for early clinical testing set forth by health authorities. Following proof of concept, our Global Drug Development unit conducts confirmatory trials on the drug candidates. In July 2018, we announced the decision to exit antibacterial and antiviral research. While the science for these programs is compelling, we decided to prioritize our resources in other areas where we believe we are better positioned to develop innovative medicines that will have a positive impact for patients. Since then, we have executed two out-licensing deals with Gilead and Boston Pharmaceuticals for assets from our infectious diseases portfolio. Regulatory Affairs and Global Development Operations, and global Development Units aligned with our business franchises. These trials also determine how a drug is absorbed, distributed, metabolized and excreted, and the duration of its action. Though we use this traditional model, we have tailored the development process to be simpler, more flexible and efficient. We divide the development process into two stages: Exploratory Development to establish proof of concept, followed by Confirmatory Development to confirm the concept in large numbers of patients. Further, with new treatment approaches such as gene therapy for rare diseases, elements of Exploratory and Confirmatory Development may be combined and suffice for registration under certain conditions such as high unmet medical need and clinical data showing highly favorable benefit-risk. In these cases, additional post-approval studies may be required by the regulatory authorities to continue to gather important data to further support approval. Information on the Company the vast amount of data that must be collected and evaluated makes clinical testing the most time-consuming and expensive part of new drug development. The next stage in the drug development process is to seek registration for the new drug. Alliances and acquisitions Our Innovative Medicines Division enters into business development agreements with other pharmaceutical and biotechnology companies and with academic and other institutions to develop new products and access new markets. We license products that complement our current product line and are appropriate to our business strategy. We focus on strategic alliances and acquisition activities for key disease areas and indications that we expect to be growth drivers in the future. We review products and compounds we are considering licensing, using the same criteria that we use for our own internally discovered drugs. This transaction supports our oncology strategy to focus on medicines that have the potential to transform the standard of care for patients in four distinct cancer treatment platforms: targeted therapies, radioligand therapies, cell and gene therapies, and immunotherapies. In October 2019, we announced a multiyear research and development collaboration with Microsoft. This alliance is expected to bolster our artificial intelligence capabilities to help accelerate the discovery, development and commercialization of medicines for patients worldwide. The new agreement allows each organization to pursue its own research in cell and gene therapies. In July 2019, we announced that we completed the acquisition of Xiidra (lifitegrast) from Takeda Pharmaceutical Company Limited, and we began recording sales as of July 1, 2019. In February 2019, we completed the acquisition of CellforCure, a French company specializing in the development and manufacture of cell and gene therapies. The pharmaceutical development and registration process is typically intensive, lengthy and rigorous. The sponsor must then submit an adequate response to the deficiencies in order to restart the review procedure. Regulatory authorities around the world administer numerous laws and regulations regarding the testing, approval, manufacturing, importing, labeling and marketing of drugs, and review the safety and efficacy of pharmaceutical products. Extensive controls exist on the non-clinical and clinical development of pharmaceutical products.